Research
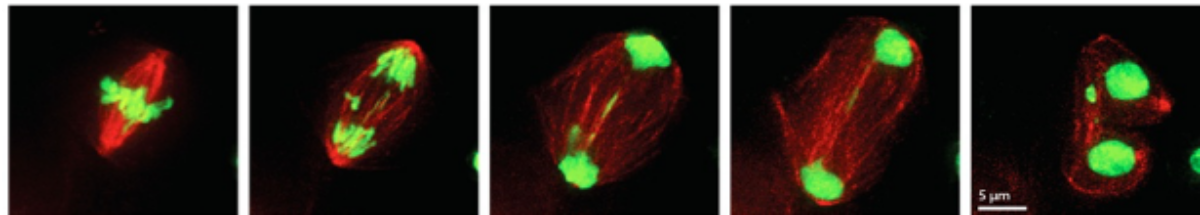
Cell division is an essential part of all live. During mitosis the duplicated chromosomes must be divided accurately to the two forming daughter cells. To do so, the chromosomes attach to spindle microtubules, which is mediated by the kinetochore that forms on centromeric chromatin on every chromosome to eventually get separated. This process is highly regulated and any mistakes can lead to aneuploidy- the loss or gain of chromosomes in a cell- a hallmark of cancer and many birth defects.
Our lab is broadly interested in epigenetic inheritance and chromatin structures with a focus on centromeric and pericentromeric chromatin and its role in chromosome segregation and genome stability. We study the regulation and function of centromeres in chromosome segregation and how its misregulation can lead to genome instability.
Our goal is to better understand how epigenetic mechanisms are linked to centromere identity and regulation, chromosome segregation, and cell division. We are thereby focusing on proteins and RNA that localize and emanate from centromeric and pericentromeric chromatin.
The fruit fly Drosophila melanogaster with its versatile genetic tools as well as human cell cultures are our primary model systems. We are using a wide range of cell and molecular biology techniques as well as biochemistry, genetics, and genome engineering to better understand centromere function and regulation.
Our current main research directions:
- Epigenetic regulation of CENP-A-containing chromatin
The histone variant CENP-A is the major epigenetic component of centromeres. It is found predominantly at active centromeric regions. We are currently focusing on CENP-A regulation and loading and how it affects cell cycle progression and genome stability. We also have a strong focus on CENP-A localization outside of centromeric chromatin and its consequences for genome stability and DNA damage repair.
- The role of (peri-) centromeric heterochromatin and transcripts in genome stability
Centromeres are embedded in large regions of highly repetitive pericentromeric chromatin. Even though these regions are built from large blocks of heterochromatin, they are transcriptionally active and highly regulated, and crucial for centromere function and genome integrity. We are currently investigating different centromeric transcripts and their function and regulation during Drosophila embryonic and germline development and human disease.